|
 -------------------
-------------------
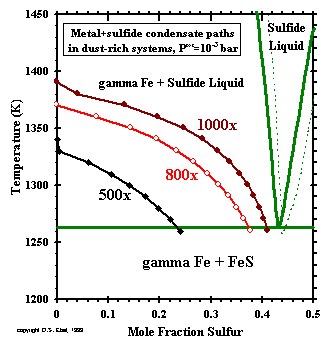
Figure 15:
Bulk chemical compositions of metallic nickel-iron + pyrrhotite condensate assemblages
predicted at Ptot= 10-3 bar
and the stated dust enrichments, projected onto the liquid-crystal phase relations of the
Fe-rich portion of the Fe-S binary system.
Dashed curves show projections of phase boundaries when 7 mole % Ni is present.
(enlarge)
-------------------
Because of the high concentration of sulfur in dust of C1 composition, enrichment in such dust leads to much
higher fS2 and permits sulfide phases to condense at higher temperatures at a
given Ptot than in a gas of solar composition. Inspection of Table 7 reveals that no sulfide phase
becomes stable above the last temperature step of the calculations at any of the dust enrichments shown
at 10-6 bar, but that, at 10-3 bar, pyrrhotite,
Fe0.877S, joins metallic nickel-iron as a stable condensate at 1330 and 1380K at dust enrichments
of 500x and 1000x, respectively. These temperatures are higher than minimum melting temperatures in the Ni-poor part of
the Fe-Ni-S system but, since our computer program does not contain a thermodynamic model for Fe-Ni-S liquids, it would
be unable to predict their existence even if they were more stable than the metal + pyrrhotite assemblages that are predicted.
In order to see if sulfide liquids are more stable than the predicted assemblages, the relative atom proportions of Fe and S
were calculated for the metallic nickel-iron + pyrrhotite assemblage predicted at each temperature step for dust enrichments
of 500x, 800x and 1000x at 10-3 bar, and are plotted on a portion of the liquid-crystal phase
relations in the Fe-S binary (Chuang et al., 1986a) in Fig. 15. The dashed curves in this figure are the phase boundaries
that result from addition of 7% Ni to the system, taken from the work of Hsieh et al. (1982), projected onto the Fe-S plane.
Under all conditions, the trajectory of the condensate compositions initially falls vertically along the left margin of the diagram
until the temperature of pyrrhotite formation is reached. Below this, the trajectories extend to the right and downward, well above
the eutectic temperature of 1262K and well within the field of gamma-iron + Fe-S liquid. The solid assemblage of metallic
nickel-iron + pyrrhotite predicted by our thermodynamic model to condense under these conditions is thus seen to form at
temperatures where it is actually metastable relative to metallic iron + Fe-S liquid. Nickel, cobalt and chromium are also
predicted to condense into the metal alloy, but the amounts of Co and Cr are quite small and the phase relations are seen to
change very little with the addition of 7% Ni, a fairly representative concentration in these condensate assemblages.
We conclude that, at these relatively high total pressures and dust enrichments, direct condensation of iron sulfide
liquids will occur. Furthermore, because the liquid-bearing assemblage obviously has a lower Gibbs free energy than
the predicted assemblage, the temperature of appearance of sulfide liquid will actually be higher than the condensation
temperature of pyrrhotite. Recall that the silicate liquid in the 500x case solidifies at 1400K, which is well above the
minimum condensation temperature of sulfide liquid, but that the silicate liquid at 1000x does not solidify until 1310K,
almost 100K below the minimum condensation temperature of sulfide liquid in this case. This means that, at the highest
dust enrichment factors at high Ptot, condensation of coexisting silicate and sulfide
liquids occurs, assuming they are not miscible.
|
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999