Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
|
------------------- Figure 11: Composition of spinel as a function of temperature at (a) Ptot= 10-3 bar and a dust enrichment of 100x; (b) Ptot= 10-3 bar and a dust enrichment of 1000x; and (c) Ptot = 10-6 bar and a dust enrichment of 1000x. Inflection points labelled as in Fig. 10, plus: b, perovskite out; c, olivine in; j, liquid out; m, MnO in; n, pyrrhotite in; r, orthopyroxene out.(enlarge a) (enlarge b) (enlarge c) |
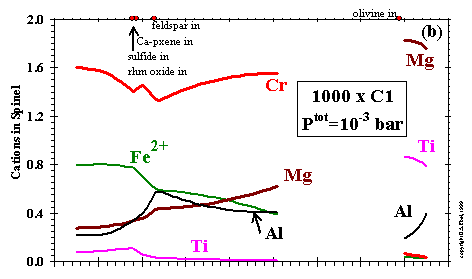 The numbers of cations in spinel per 4 oxygen atoms are plotted as a function of temperature at dust enrichments
of 100x and 1000x at 10-3 bar in Figs. 11a and 11b, respectively, and 1000x
at 10-6 bar in Fig. 11c. In all cases, the highest temperature spinel forms by reaction of gaseous Mg
with TiO2 in perovskite and Al2O3 in
the CMAS liquid, except at 1000x and 10-3 bar, where all Ti is from the gas. In this spinel, the Ti
cations first increase with falling temperature as perovskite and/or gaseous Ti are consumed and then decrease sharply when the MELTS
liquid, which can accommodate Ti, becomes stable. Both stages proceed in accordance with the coupled substitution
of Mg2+ + Ti4+
= 2Al3+, and
are accompanied by steadily rising numbers of Fe and Cr cations which are condensing from the gas. As discussed previously, the high
Ti contents of these spinels and possibly even their existence, may be artifacts of the inability of the CMAS liquid to accommodate Ti.
The incoming of the MELTS liquid causes the very Ti-rich spinel at 1000x and 10-3 bar to dissolve suddenly,
and the less Ti-rich spinel at 100x and 10-3 bar to dissolve gradually before disappearing. The even lower-Ti
spinel at 1000x and 10-6 bar continues to crystallize with falling temperature, gradually becoming first more
Cr-rich and then more Fe-rich.
The numbers of cations in spinel per 4 oxygen atoms are plotted as a function of temperature at dust enrichments
of 100x and 1000x at 10-3 bar in Figs. 11a and 11b, respectively, and 1000x
at 10-6 bar in Fig. 11c. In all cases, the highest temperature spinel forms by reaction of gaseous Mg
with TiO2 in perovskite and Al2O3 in
the CMAS liquid, except at 1000x and 10-3 bar, where all Ti is from the gas. In this spinel, the Ti
cations first increase with falling temperature as perovskite and/or gaseous Ti are consumed and then decrease sharply when the MELTS
liquid, which can accommodate Ti, becomes stable. Both stages proceed in accordance with the coupled substitution
of Mg2+ + Ti4+
= 2Al3+, and
are accompanied by steadily rising numbers of Fe and Cr cations which are condensing from the gas. As discussed previously, the high
Ti contents of these spinels and possibly even their existence, may be artifacts of the inability of the CMAS liquid to accommodate Ti.
The incoming of the MELTS liquid causes the very Ti-rich spinel at 1000x and 10-3 bar to dissolve suddenly,
and the less Ti-rich spinel at 100x and 10-3 bar to dissolve gradually before disappearing. The even lower-Ti
spinel at 1000x and 10-6 bar continues to crystallize with falling temperature, gradually becoming first more
Cr-rich and then more Fe-rich.
|
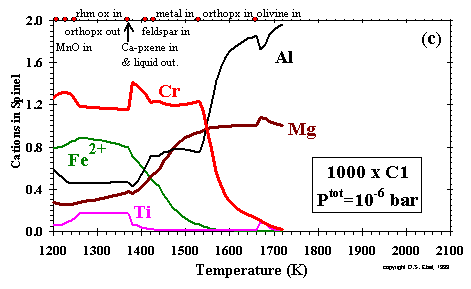 As shown in Fig. 11c, the Cr/Al ratio levels off below 1390K, as formation of spinel continues by reaction of
gaseous Cr with Al2O3 in the liquid. When spinel re-forms in the two cases
at 10-3 bar, its Cr/Al ratio falls, as the spinel draws
down Al2O3 from the liquid while deriving its Cr from the metal alloy and
the gas at 100x, and from the metal alloy and the liquid at 1000x. In all three cases, these trends are interrupted by plagioclase formation,
which draws its Al2O3 from
the MgAl2O4 component of the spinel, increasing the Cr/Al ratio and decreasing
the amount of spinel. Plagioclase formation also causes an increase in the rate of increase of the number of Ti cations in the spinel with
decreasing temperature, accompanied by an increase in the number of Mg and/or Fe ions in accordance with the above coupled substitution.
At lower temperature, the number of Ti ions in the spinel begins to decrease with decreasing temperature due to extraction of Ti into pyrophanite
or, at 100x and 10-3 bar, clinopyroxene.
As shown in Fig. 11c, the Cr/Al ratio levels off below 1390K, as formation of spinel continues by reaction of
gaseous Cr with Al2O3 in the liquid. When spinel re-forms in the two cases
at 10-3 bar, its Cr/Al ratio falls, as the spinel draws
down Al2O3 from the liquid while deriving its Cr from the metal alloy and
the gas at 100x, and from the metal alloy and the liquid at 1000x. In all three cases, these trends are interrupted by plagioclase formation,
which draws its Al2O3 from
the MgAl2O4 component of the spinel, increasing the Cr/Al ratio and decreasing
the amount of spinel. Plagioclase formation also causes an increase in the rate of increase of the number of Ti cations in the spinel with
decreasing temperature, accompanied by an increase in the number of Mg and/or Fe ions in accordance with the above coupled substitution.
At lower temperature, the number of Ti ions in the spinel begins to decrease with decreasing temperature due to extraction of Ti into pyrophanite
or, at 100x and 10-3 bar, clinopyroxene.
|