Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
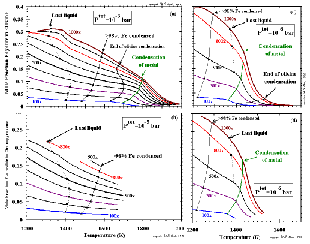
------------------ Figure 8: Mole fraction of iron end-member in olivine at (a) at 10-3 bar and (c) 10-6 bar and in orthopyroxene at (b) 10-3 bar and (d) 10-6 bar as a function of temperature and at dust enrichment factors with integral multiples of 100. Trajectories of the condensation temperature of metallic nickel-iron alloy, the temperature at which direct condensation of olivine ceases, the temperature at which >98% of the total iron is condensed, and the temperature of disappearance of silicate liquid are indicated. (enlarge a) (enlarge b) (enlarge c) (enlarge d) |
| The distributions of Al, Ca, Mg, Si, Fe, and Na and K between condensed phases and vapor are illustrated in Figs. 7a, b, c, d, e, and f, respectively, for a system enriched 1000x in dust at Ptot=10-3 bar. At a dust enrichment of 1000x, a CMAS liquid is already present at 2400K, into which 75% of the Al and 27% of the Ca have condensed. At 2050K, gaseous Ti, Mg, Cr and Fe begin to react with Al2O3 in the liquid to form a spinel containing 44.8 wt% MgO, 39.5% TiO2, 12.7% Al2O3, 1.8% Cr2O3, 1.1% FeO and 0.12% Fe2O3. At 1990K, olivine begins to form primarily by condensation from the gas but some also by crystallization from the liquid, and the switch is made from the CMAS to the MELTS liquid model which, because the latter can accommodate TiO2, causes the titanian spinel to dissolve into the liquid. The initial olivine contains 0.92 wt% FeO and 0.23% CaO but, as Fe and Mg condense into it, reaches 10.8% FeO and 0.13% CaO by 1800K. Over the same temperature range, as Fe and Cr condense into the liquid, the composition of the latter evolves from 0.73 wt% FeO, 0.39% TiO2, 0.02% Cr2O3 and <0.01% Fe2O3 to 24.2% FeO, 0.23% TiO2, 0.90% Cr2O3 and 0.19% Fe2O3. Metal alloy containing 20.0 wt% Ni, 0.70% Co and 0.09% Cr begins to condense at 1800K, and reaches 11.7% Ni, 0.52% Co and 0.06% Cr by 1720K. In this temperature range, olivine continues to form at the expense of liquid and reaches 14.3 wt% FeO and 0.13% CaO, while the liquid composition evolves to 25.9 wt% FeO, 0.25% TiO2, 1.22% Cr2O3 and 0.16% Fe2O3. At 1710K, a small amount of Cr-spinel begins to form by drawing Al and most of its Cr from the liquid but some of its Cr also from the gas and the metal. Initially, it has molar Fe/Mg and Cr/Al ratios of 0.62 and 3.79, respectively, and contains 1.10 wt% Fe2O3 and 0.27% TiO2 but varies in composition as it continues to crystallize from the liquid with falling temperature, reaching molar Fe/Mg and Cr/Al ratios of 1.34 and 2.29, respectively, with 1.42 wt% Fe2O3 and 1.07% TiO2 at 1440K. Over the same temperature range, the amount of olivine continues to increase with falling temperature, drawing its MgO, SiO2 and CaO from the liquid. From 1710 to 1560K, the FeO consumed by olivine comes from both liquid and gas but, at 1560K, the temperature below which <1% of the Fe remains in the gas, oxidation of the metal alloy joins the liquid as a source of the FeO for continued production of olivine. The amount of metal alloy increases with falling temperature from 1710 to 1560K, as gaseous Fe continues to condense into it, diluting its Ni, Co and Cr concentrations to 9.3% Ni, 0.43% Co and 0.01% Cr at 1560K. Below 1560K, however, oxidation of Fe causes the amount of metal to decrease with falling temperature, increasing its Ni and Co contents to 10.0 and 0.46 wt%, respectively, by 1440K. Its Cr content continues to decrease due to formation of increasing amounts of Cr-spinel with falling temperature. Olivine contains 21.1 wt% FeO and 0.24% CaO at 1560K, and 24.6% FeO and 0.45% CaO at 1440K. As the amount of liquid decreases with falling temperature, its FeO, Cr2O3 and Fe2O3 contents progressively decrease, reaching 17.6 wt%, 0.45% and 0.02%, respectively, at 1560K and 10.8%, 0.12% and 0.01% at 1440K; and its TiO2, Na2O, K2O and P2O5 contents progressively increase, reaching 0.43 wt%, 0.29%, 0.10% and <0.01%, respectively, at 1560K and 0.56%, 2.79%, 0.35% and 0.20% at 1440K. At 1480K, MnO condenses and, at 1430K, plagioclase containing 34.4 mole % albite and 0.35% orthoclase begins to crystallize from the liquid. As the amount of plagioclase increases with falling temperature, its albite and orthoclase contents also increase. Although the Na required for this is supplied by both gas and liquid, the K is derived only from the liquid, with the proportion of K residing in the vapor actually increasing initially with falling temperature. At 1390K, a diopside-rich clinopyroxene, containing 4.7 wt% FeO, 2.3% Al2O3, 0.18% Na2O, 0.36% TiO2 and 0.09% Fe2O3, crystallizes from the liquid. At 1380K, a pyrophanite-rich oxide solid solution, containing 9.53 wt% FeO, 1.02% MgO and 0.52% Fe2O3, forms by reaction of gaseous Mn with TiO2 in the liquid; and gaseous sulfur begins to react with metallic Fe to form pyrrhotite, Fe0.877S. The concentrations of Ni and Co in the residual alloy are 11.5 wt% and 0.53%, respectively, but increase sharply as more pyrrhotite forms with falling temperature, reaching 21.4% and 0.98%, respectively, at 1310K. At 1350K, gaseous P reacts with the liquid to form whitlockite. At 1320K, just before disappearing, the liquid contains 10.1 wt% Na2O, 4.52% FeO, 1.34% K2O, 0.77% P2O5, 0.41% TiO2 and 0.02% Cr2O3. At 1260K, olivine contains 27.3 wt% FeO and 0.31% CaO; clinopyroxene contains 4.5 wt% FeO, 1.6% Al2O3, 0.33% Na2O, 0.33% TiO2, and 0.07% Fe2O3; Cr-spinel has molar Fe/Mg and Cr/Al ratios of 2.9 and 7.5, respectively, and contains 2.8 wt% TiO2 and 1.5% Fe2O3; the pyrophanite-rich solid solution contains 9.2 wt% FeO, 0.86% MgO and 0.45% Fe2O3; and the metal alloy contains 27.5 wt% Ni and 1.3% Co. At this point, 97.8% of the P is condensed as whitlockite, 65.1% of the sulfur as pyrrhotite, and 90.0% of the Na and 65.5% of the K as feldspar. The ratio of the proportion of Fe in sulfide to that in metal is 2.2. |