Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
| Anticipating that condensate liquids will be poor in non-CMAS components and will thus violate caveat (a), we tested MELTS calculations against quenched partial melting experiments of peridotite KLB-1, whose non-CMAS components consist of only 8.1 wt% FeO, and (> or =) 0.3% of all other oxides. Takahashi (1986) and Takahashi et al. (1993) reported the temperature intervals between the observed absence and presence of phases, as well as phase compositions and melt fractions for KLB-1 at 1 bar at the Ni-NiO oxygen buffer. Note that only one of their seven data points used here is used in the MELTS calibration database. | |
|
------------------- Figure 1: Comparison of melt fractions measured in peridotite melting experiments of Takahashi (1986) and Takahashi et al. (1993) with those calculated at 1 bar using MELTS. (enlarge) |
|
|
It can be seen in Fig. 1 that the MELTS model reproduces the observed volume fractions of liquids well, except at
low melt fractions where there may be significant measurement error in the experiments. The solidus temperature and appearance temperatures of olivine,
Ca-pyroxene, and feldspar agree nearly within experimental error, but the model underpredicts the crystallization temperature of orthopyroxene and
overpredicts that of spinel (Table 5). Hirschmann et al. (1998) observed that the MELTS model overpredicted the crystallization temperature
of orthopyroxene at 10 kbar. These differences reflect compromises made by Sack and Ghiorso (1994c) to best satisfy both high- and low-pressure pyroxene-liquid
phase relations.
|
|
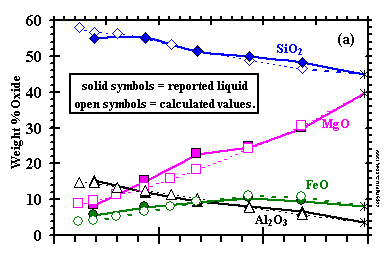 In Fig. 2,
the 1 bar liquid compositions are compared with MELTS results, with all Fe2O3
recalculated to FeO. The good agreement of the results for melt fraction and composition suggests that the MELTS model will yield reasonably accurate results in
the condensation calculation, particularly because olivine dominates the distribution of mass in condensation sequences. Because spinel is a minor phase,
overstabilization of spinel will not have a significant effect on liquid stability.
In Fig. 2,
the 1 bar liquid compositions are compared with MELTS results, with all Fe2O3
recalculated to FeO. The good agreement of the results for melt fraction and composition suggests that the MELTS model will yield reasonably accurate results in
the condensation calculation, particularly because olivine dominates the distribution of mass in condensation sequences. Because spinel is a minor phase,
overstabilization of spinel will not have a significant effect on liquid stability.
|
|
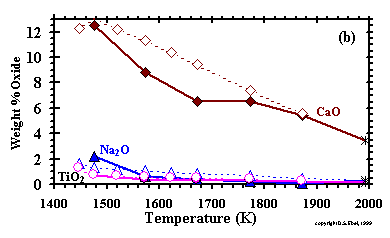 The understabilization of orthopyroxene, relative to liquid, suggests
that liquid stability might be slightly overpredicted when orthopyroxene condenses with it, and that the temperature of appearance of the latter phase in
the condensation calculation may be too low.
The understabilization of orthopyroxene, relative to liquid, suggests
that liquid stability might be slightly overpredicted when orthopyroxene condenses with it, and that the temperature of appearance of the latter phase in
the condensation calculation may be too low. | |
| Figure 2: Comparison of measured compositions of KLB-1 liquids (Takahashi, 1986; Takahashi et al., 1993) with those calculated from MELTS, all at one bar. Asterisks indicate starting compositions in the experiments. (enlarge 2a) (enlarge 2b) |
| In addition to MELTS, Berman's (1983) model for CaO-MgO-Al2O3-SiO2(CMAS) liquids is included in the present work. Yoneda and Grossman (1995) used this model, and explained in detail its advantages and drawbacks. The CMAS liquid model works well at high temperatures, where these four oxides are the only major ones condensed, but it is inadequate under conditions where FeO, Na2O and other non-CMAS components condense in appreciable quantities. Therefore, the MELTS model must be used at lower temperatures where non-CMAS oxides are important constituents of the liquid. The purely CMAS liquid region is very far outside the composition range over which MELTS is calibrated, and contains too few components for reliable application of the MELTS liquid model. Furthermore, because the MELTS liquid uses mostly silicate components, not pure oxides, as end-members, it cannot be applied to some especially Ca- and Al-rich regions of composition space that are treated adequately by the CMAS liquid model. For example, CaSiO3 is the major Ca-containing component employed by MELTS; yet, early high temperature condensate liquids never contain as much SiO2 as CaO. For these reasons, both models are required to completely describe condensation of silicate liquids over the temperature ranges where liquids may be stable. |