Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
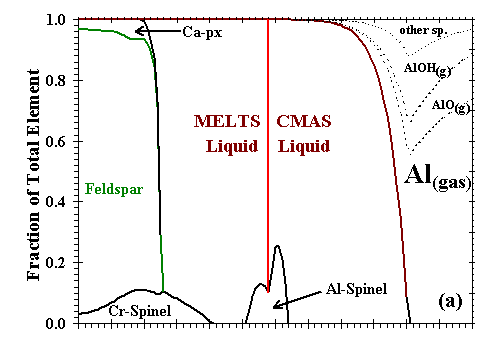 Figure 6: Distribution of (a) Al; (b) Ca; (c) Mg; (d) Si; (e) Fe; and (f) Na and K between condensed phases and vapor at a dust enrichment of 100x
at Ptot=10-3 bar. Note vertical scale change in (e).
Al-spinel=spinel with (> or =) 1 wt% Cr2O3;
Cr-spinel=spinel with >1 wt% Cr2O3.
Other abbreviations as used previously.
Figure 6: Distribution of (a) Al; (b) Ca; (c) Mg; (d) Si; (e) Fe; and (f) Na and K between condensed phases and vapor at a dust enrichment of 100x
at Ptot=10-3 bar. Note vertical scale change in (e).
Al-spinel=spinel with (> or =) 1 wt% Cr2O3;
Cr-spinel=spinel with >1 wt% Cr2O3.
Other abbreviations as used previously.
|

|
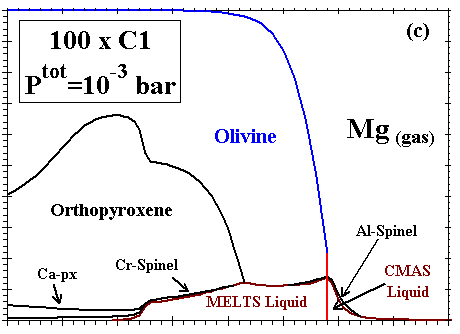 Figure 6: Distribution of (a) Al; (b) Ca; (c) Mg; (d) Si; (e) Fe; and (f) Na and K between condensed phases and vapor at a dust enrichment of 100x
at Ptot=10-3 bar. Note vertical scale change in (e).
Al-spinel=spinel with (> or =) 1 wt% Cr2O3;
Cr-spinel=spinel with >1 wt% Cr2O3.
Other abbreviations as used previously.
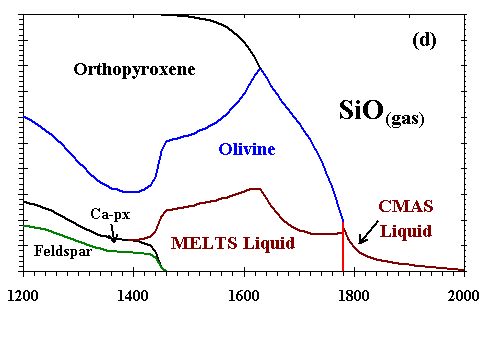
Figure 6: Distribution of (a) Al; (b) Ca; (c) Mg; (d) Si; (e) Fe; and (f) Na and K between condensed phases and vapor at a dust enrichment of 100x
at Ptot=10-3 bar. Note vertical scale change in (e).
Al-spinel=spinel with (> or =) 1 wt% Cr2O3;
Cr-spinel=spinel with >1 wt% Cr2O3.
Other abbreviations as used previously.
|
|
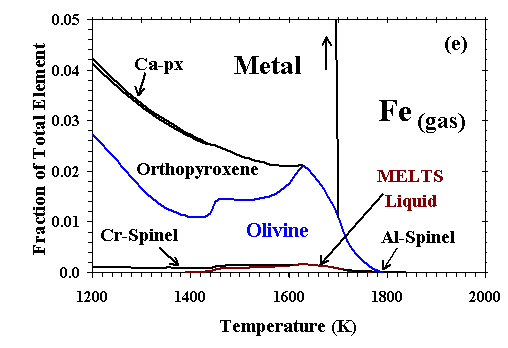 Figure 6: Distribution of (a) Al; (b) Ca; (c) Mg; (d) Si; (e) Fe; and (f) Na and K between condensed phases and vapor at a dust enrichment of 100x
at Ptot=10-3 bar. Note vertical scale change in (e).
Al-spinel=spinel with (> or =) 1 wt% Cr2O3;
Cr-spinel=spinel with >1 wt% Cr2O3.
Other abbreviations as used previously.
Figure 6: Distribution of (a) Al; (b) Ca; (c) Mg; (d) Si; (e) Fe; and (f) Na and K between condensed phases and vapor at a dust enrichment of 100x
at Ptot=10-3 bar. Note vertical scale change in (e).
Al-spinel=spinel with (> or =) 1 wt% Cr2O3;
Cr-spinel=spinel with >1 wt% Cr2O3.
Other abbreviations as used previously.
|
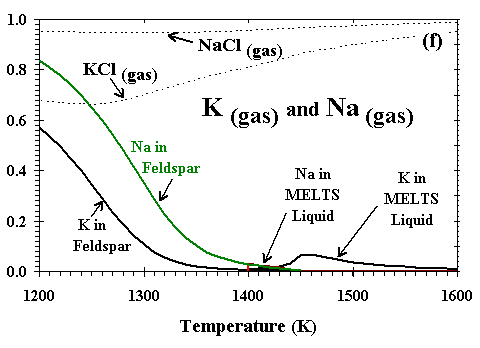
|
| The distributions of Al, Ca, Mg, Si, Fe, and Na and K between condensed phases and vapor are illustrated in Figs. 6a, b, c, d, e, and f, respectively, for a system enriched 100x in dust at Ptot=10-3 bar. The first condensate, a CMAS liquid extremely rich in Al2O3 (79 mole %) and CaO (21%) with only minor amounts of MgO and SiO2, condenses at 2200K. Perovskite becomes stable at 1970K, and consumes 85% of the total Ti in the system by 1850K. At 1830K, gaseous Mg, Fe and Cr begin to react with the liquid and perovskite to form an Mg-, Al-rich spinel with minor amounts of Cr, Fe and Ti. By 1810K, this spinel has molar Fe/Mg and Cr/Al ratios of 3.4x10-3 and 1.3x10-2, respectively, and a TiO2 content of 9.1 wt%, so much Ti that perovskite disappears at this temperature. With falling temperature, Ti and Mg continue to condense into spinel, and Si and Mg into the liquid. The SiO2 and MgO contents of the liquid increase, and the MgAl2O4 component of the spinel dissolves into the liquid. By 1780K, the liquid reaches ~40 wt% SiO2 and ~26% MgO, olivine (0.25 wt% FeO, 0.73% CaO) becomes stable and, as discussed above, this triggers the switchover from the CMAS to the MELTS liquid model. At this point, the spinel has molar Fe/Mg and Cr/Al ratios of 3.0x10-3 and 2.3x10-2, respectively, and, because Ti can now be accommodated by the liquid model, the TiO2 content of the liquid is 0.8 wt% and Ti drops to only 0.44 wt% in the spinel. With falling temperature, most olivine forms by wholesale condensation of Mg and Si from the gas but some by crystallization of the liquid. With the total amount of liquid decreasing slightly, the MgAl2O4 component of spinel continues to dissolve into it, causing the molar Fe/Mg and Cr/Al ratios in spinel to rise to 9.0x10-3 and 6.2x10-2, respectively, just before spinel dissolves completely into the liquid at 1710K. With falling temperature, the FeO content of olivine increases and its CaO decreases, reaching 0.89 wt% and 0.48%, respectively, at the point where spinel disappears and 1.29% and 0.42%, respectively, at 1690K, the initial condensation temperature of metallic NiFe. This alloy contains 13.6 wt% Ni, 0.48% Co and 0.24% Cr. As the temperature falls, olivine of increasing FeO and decreasing CaO content continues to condense from the gas, Si and Fe continue to condense into the liquid, diluting its MgO, Al2O3 and CaO contents, and metal of decreasing Ni and Co and increasing Cr content continues to condense. At 1620K, when nearly all the Mg and 80% of the Si are condensed, gaseous SiO begins to react with olivine and liquid to form orthopyroxene whose initial FeO content is 1.1 wt%. At this point, olivine contains 1.9 wt% FeO and 0.23% CaO and the liquid contains 0.59 wt% TiO2, 0.23% Cr2O3 and 0.30% FeO. At 1600K, gaseous Cr begins to react with the liquid to form a small amount of Cr-spinel, having molar Fe/Mg and Cr/Al ratios of 0.049 and 2.1, respectively, and a TiO2 content of 0.26 wt%. With falling temperature, orthopyroxene of increasing FeO content continues to form by reaction of gaseous SiO with liquid and with olivine of increasing FeO and CaO contents; all components of the metal alloy continue to condense, resulting in decreasing Ni, Co and Cr contents; the amount of Cr-spinel continues to increase at the expense of Al2O3 in the liquid and gaseous Cr; and the CaO and TiO2 contents of the liquid increase while its FeO and Cr2O3 contents decrease. By 1550K, 1.8% of the K has condensed into the liquid which contains 0.01wt% K2O. At 1450K, 99% of the Fe is condensed, and gaseous Na begins to react with the liquid to form plagioclase feldspar containing 1.6 mole % albite. At this point, olivine contains 2.30 wt% FeO and 0.34% CaO, Cr-spinel has molar Fe/Mg and Cr/Al ratios of 0.053 and 1.43, respectively, and 0.34 wt% TiO2, and liquid contains 0.93 wt% TiO2, 0.20% Cr2O3, 0.19% FeO, 0.06% K2O and 0.01% Na2O. At 1440K, a diopsidic clinopyroxene begins to co-crystallize with feldspar from the remaining liquid, which reaches 0.15 wt% K2O and 0.03% Na2O before disappearing at 1390K. With continued cooling, gaseous Na and K react with anorthitic feldspar, increasing its albite and orthoclase contents and displacing Ca which, in turn, reacts with orthopyroxene to form clinopyroxene and olivine. The FeO contents of olivine, orthopyroxene, clinopyroxene and spinel all increase as metallic Fe becomes oxidized, causing the Ni and Co contents of the alloy to increase even as its Cr content decreases due to reaction with spinel. At 1350K, gaseous Mn reacts with Ti in the spinel and clinopyroxene to form an oxide solid solution consisting of pyrophanite [MnTiO3] with 9.4 wt% MgO and 2.6% FeO. At 1300K, the remaining gaseous Mn begins to condense as MnO. By 1200K, olivine contains 3.6 wt% FeO and 0.10% CaO; clinopyroxene contains 1.4% Al2O3, 0.62% FeO, 0.25% TiO2, and 0.27% Na2O; spinel has molar Fe/Mg and Cr/Al ratios of 0.13 and 5.1, respectively, and a TiO2 content of 0.59%; and the oxide solid solution contains only 1.3% MgO and 1.6% FeO. At this temperature, Na, K and Mn are only partially condensed, with 15, 43 and 3.5%, respectively, remaining in the vapor. |