Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
|
------------------- Figure 11: Composition of spinel as a function of temperature at (a) Ptot= 10-3 bar and a dust enrichment of 100x; (b) Ptot= 10-3 bar and a dust enrichment of 1000x; and (c) Ptot = 10-6 bar and a dust enrichment of 1000x. Inflection points labelled as in Fig. 10, plus: b, perovskite out; c, olivine in; j, liquid out; m, MnO in; n, pyrrhotite in; r, orthopyroxene out.(enlarge a) (enlarge b) (enlarge c) |
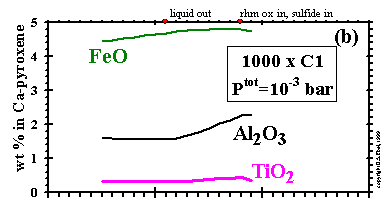 The concentrations of FeO, Al2O3
and TiO2 in clinopyroxene are plotted as a function of temperature
at 10-3 bar and dust enrichments of 100x and 1000x, and
at 10-6 bar and 1000x in Figs. 12a, b and c, respectively.
The amount of clinopyroxene increases with falling temperature in all three cases due either to
crystallization from the liquid or, after liquid is exhausted, to reactions among plagioclase,
orthopyroxene and olivine, as can be seen in Figs. 6 and 7 for the cases
at 10-3 bar.
The concentrations of FeO, Al2O3
and TiO2 in clinopyroxene are plotted as a function of temperature
at 10-3 bar and dust enrichments of 100x and 1000x, and
at 10-6 bar and 1000x in Figs. 12a, b and c, respectively.
The amount of clinopyroxene increases with falling temperature in all three cases due either to
crystallization from the liquid or, after liquid is exhausted, to reactions among plagioclase,
orthopyroxene and olivine, as can be seen in Figs. 6 and 7 for the cases
at 10-3 bar.
|
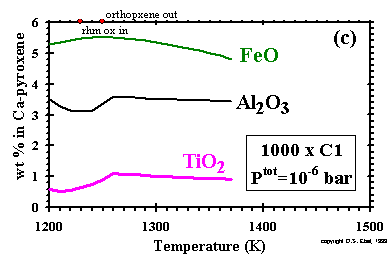 The proportion of the total Fe accounted for by
clinopyroxene increases with falling temperature as metal is oxidized, but the concentration
of FeO may rise or fall depending on the relative rates of formation of Mg and Fe end-members.
Similarly, the proportions of the total Al and Ti accounted for by clinopyroxene increase with
falling temperature as this phase crystallizes from the liquid in the cases
at 10-3 bar, but the concentrations
of Al2O3 and
TiO2 may increase or decrease with falling temperature due to the relative
formation rates of the different pyroxene end-members. At 10-3 bar and
100x and at 10-6 bar and 1000x, a temperature is reached below which the
Al2O3 and
TiO2 concentrations begin to fall with decreasing
temperature, as plagioclase begins to draw its
Al2O3, and pyrophanite its
TiO2, from clinopyroxene.
The proportion of the total Fe accounted for by
clinopyroxene increases with falling temperature as metal is oxidized, but the concentration
of FeO may rise or fall depending on the relative rates of formation of Mg and Fe end-members.
Similarly, the proportions of the total Al and Ti accounted for by clinopyroxene increase with
falling temperature as this phase crystallizes from the liquid in the cases
at 10-3 bar, but the concentrations
of Al2O3 and
TiO2 may increase or decrease with falling temperature due to the relative
formation rates of the different pyroxene end-members. At 10-3 bar and
100x and at 10-6 bar and 1000x, a temperature is reached below which the
Al2O3 and
TiO2 concentrations begin to fall with decreasing
temperature, as plagioclase begins to draw its
Al2O3, and pyrophanite its
TiO2, from clinopyroxene.
|