Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
|
The progressive increase in condensation temperatures of all phases with increasing dust enrichment at constant Ptot, as seen by Yoneda and Grossman (1995), is illustrated in Tables 7 and 8. At 10-6 bar, condensation temperatures are still low enough at a dust enrichment of 100x that no liquid phase is stable. At this Ptot and above a dust enrichment between 400x and 450x, however, the oxide + silicate fraction of the assemblage that condenses in certain temperature intervals does so at a temperature above the solidus temperature for its bulk chemical composition, causing liquid to be a stable condensate. Upon cooling a system at 10-6 bar and a dust enrichment of 500x, liquid first appears at 1740K, where melilite and CaAl2O4 react with the gas to form grossite and a CMAS liquid. This liquid field persists for only 10K, at which point it crystallizes into melilite and grossite. A liquid field reappears at 1630K by reaction of melilite with the gas, and persists to 1390K. At 10-6 bar and a dust enrichment of 1000x, condensation of all phases occurs at even higher temperatures such that a much greater range of bulk condensate compositions forms above solidus temperatures, causing the liquid stability field to extend to higher temperature, 1880K, and to persist without interruption to 1370K, replacing the stability fields of CaAl2O4, melilite and grossite. At constant dust enrichment, condensation temperatures of all phases are higher at 10-3 bar than at 10-6 bar because partial pressures of most condensable elements increase with Ptot. As a result, the minimum dust enrichment necessary to condense partial melts at 10-3 bar is considerably lower than at 10-6 bar, and lies between 12x and 13x. At 10-3 bar, there is, at a dust enrichment of only 100x, an extensive and uninterrupted stability field of liquid extending up to 2200K and replacing the stability fields of corundum, hibonite, grossite, CaAl2O>4 and melilite. At higher dust enrichments at this Ptot, the liquid stability field extends to even higher temperatures. |
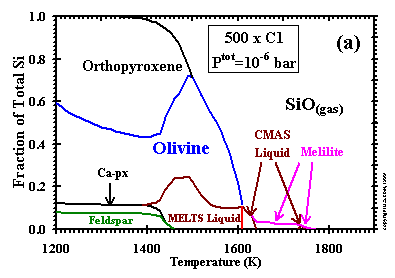
------------------- Figure 4: Distribution of Si between condensed phases and gas at Ptot = 10-6 bar and a dust enrichment of (a) 500x; and (b) 1000x. Ca-px=Ca-rich clinopyroxene. |
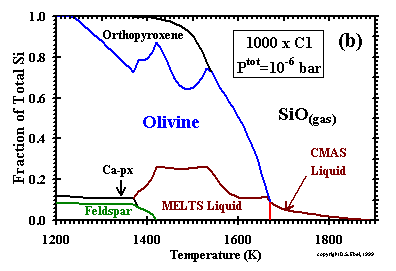 One way of viewing trends in the size of the liquid stability field as a function of Ptot and dust enrichment is by comparison
of graphs of the distribution of silicon between condensed phases and vapor vs. temperature. Such diagrams are presented in Figs. 5a and b
for 10-6 bar and dust enrichments of 500x and 1000x, respectively, and should be compared to Figs. 6d and 7d for
the cases of 100x and 1000x, respectively, at 10-3 bar. At 10-3 bar, the liquid has a
stability field 800K wide (Table 8) and accounts for a maximum of 32% of the silicon at a dust enrichment of 100x. At the same Ptot,
the liquid field widens to >1100K, with a maximum of 60% of the silicon, at a dust enrichment of 1000x. Although both the temperature interval for the
stability of liquid and the maximum fraction of the total silicon accounted for by the liquid are always smaller at 10-6 bar
than at 10-3 bar for the same dust enrichment, the liquid fields at 10-6 bar are still quite
extensive at these elevated dust enrichments. For example, although a very small, high-temperature field of liquid is separated from a lower-temperature
liquid field at 500x, the latter field is 240K wide and accounts for a maximum of 25% of the silicon and, at 1000x, the liquid field is over 500K wide and
accounts for a maximum of 26% of the silicon. Petaev and Wood (1998) found no liquid stability field at 10-5 bar and a dust enrichment
of 107. This is in clear disagreement with the results of Wood and Hashimoto (1993) who found a small liquid stability
field at the same Ptot and a lower dust enrichment, 103, as both studies employed the same liquid
solution model and dust composition. The Petaev and Wood (1998) results are also in complete disagreement with ours in that we find a liquid stability
field at both lower Ptot and lower dust enrichment. Although the existence of a liquid stability field in Wood and
Hashimoto’s (1993) study is in general agreement with the work presented here, both the amount of liquid and the temperature interval of its stability are
much smaller than would be expected from our work, presumably because of Wood and Hashimoto’s (1993) use of an ideal solution model for silicate liquids,
which are demonstrably non-ideal (Berman, 1983; Ghiorso et al., 1983), and the difference between their assumed dust composition and ours.
One way of viewing trends in the size of the liquid stability field as a function of Ptot and dust enrichment is by comparison
of graphs of the distribution of silicon between condensed phases and vapor vs. temperature. Such diagrams are presented in Figs. 5a and b
for 10-6 bar and dust enrichments of 500x and 1000x, respectively, and should be compared to Figs. 6d and 7d for
the cases of 100x and 1000x, respectively, at 10-3 bar. At 10-3 bar, the liquid has a
stability field 800K wide (Table 8) and accounts for a maximum of 32% of the silicon at a dust enrichment of 100x. At the same Ptot,
the liquid field widens to >1100K, with a maximum of 60% of the silicon, at a dust enrichment of 1000x. Although both the temperature interval for the
stability of liquid and the maximum fraction of the total silicon accounted for by the liquid are always smaller at 10-6 bar
than at 10-3 bar for the same dust enrichment, the liquid fields at 10-6 bar are still quite
extensive at these elevated dust enrichments. For example, although a very small, high-temperature field of liquid is separated from a lower-temperature
liquid field at 500x, the latter field is 240K wide and accounts for a maximum of 25% of the silicon and, at 1000x, the liquid field is over 500K wide and
accounts for a maximum of 26% of the silicon. Petaev and Wood (1998) found no liquid stability field at 10-5 bar and a dust enrichment
of 107. This is in clear disagreement with the results of Wood and Hashimoto (1993) who found a small liquid stability
field at the same Ptot and a lower dust enrichment, 103, as both studies employed the same liquid
solution model and dust composition. The Petaev and Wood (1998) results are also in complete disagreement with ours in that we find a liquid stability
field at both lower Ptot and lower dust enrichment. Although the existence of a liquid stability field in Wood and
Hashimoto’s (1993) study is in general agreement with the work presented here, both the amount of liquid and the temperature interval of its stability are
much smaller than would be expected from our work, presumably because of Wood and Hashimoto’s (1993) use of an ideal solution model for silicate liquids,
which are demonstrably non-ideal (Berman, 1983; Ghiorso et al., 1983), and the difference between their assumed dust composition and ours.
From Tables 7 and 8, it is clear that the assemblage liquid + metallic nickel-iron + olivine + orthopyroxene + Cr-spinel occupies a very wide stability field within the ranges of Ptot and dust enrichment considered herein. |