Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
| A decision must be made as to when to switch from one model to the other. In order to model non-CMAS oxides in the liquid, it would be best to switch to the MELTS model at the highest feasible temperature. |
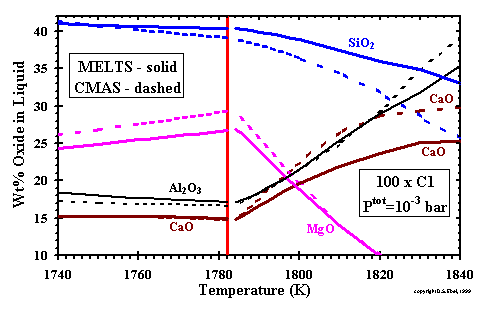
------------------- Figure 3: Compositions of CMAS and MELTS liquids near the olivine appearance temperature (vertical line at 1782 K) at the stated conditions. In this temperature range, the MELTS liquid also contains ~1 wt% of other oxides, which are not shown. (enlarge) |
| For the case of 100x dust enrichment at Ptot=10-3 bar, the curves in Fig. 3 illustrate the major oxide compositions of the two liquids, calculated at 2K intervals, near the appearance temperature of olivine, indicated by the vertical line at 1782K. The CMAS liquid is CaO- and Al2O3-rich at high temperatures, but SiO2 and MgO increase rapidly with decreasing temperature. By contrast, although a MELTS liquid becomes stable well above 1782K, it is CaO-deficient and SiO2-enriched, relative to the CMAS liquid, because the only liquid the MELTS model can determine to be stable must have sufficient SiO2 to supply the required CaSiO3 component. That is, in the temperature range above at least 1790K, the most stable liquid possible in the MELTS composition range is not the liquid which should be stable. When the temperature of olivine appearance is reached, however, the MELTS liquid has gained sufficient SiO2 and MgO to have a composition very similar to the CMAS liquid at the same temperature. Once sufficient SiO2 has condensed, the MELTS model closely tracks the Berman (1983) model liquid, but also accounts for increasing FeO and TiO2 contents. |