Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
|
------------------- Figure 9: Concentrations of (a) major and (b) minor components of the total condensate as a function of temperature at the stated conditions. Filled circles indicate average composition of H-Group chondrites.(enlarge a) (enlarge b) |
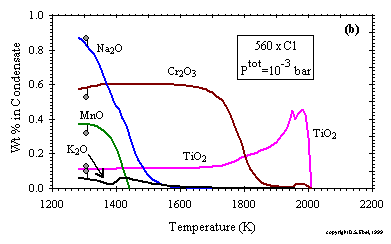 In Fig. 9a and b, the bulk chemical composition of the total condensate is plotted as a function of temperature at a
Ptot of 10-3 bar and a dust enrichment of 560x. Features of this diagram which are
common to condensation at all dust enrichments are the early entry of Al, Ca and Ti relative to Mg and Si, as well as the relatively late entry of
Na, K and Mn into the condensates. Features specific to condensation at this dust enrichment are the relative proportions of metal, FeO and sulfide
as a function of temperature. This particular dust enrichment was chosen because it yields a single temperature at which the distribution of Fe
between metal, sulfide and silicate matches closely the distribution found in H-Group ordinary chondrites and results in a bulk chemical composition
very close to the average of those meteorites. For example, at 1310K, the total condensate and, for comparison (brackets), the average H-Group chondrite
fall from Jarosewich (1990) contains 18.8 (17.8) wt % Fe+Ni+Co metal, 10.2 (10.3) % FeO, 5.9 (5.4) % FeS, 34.2 (36.6) % SiO2
and 24.6 (23.3) % MgO. Similarly, at the same Ptot and a slightly higher dust enrichment of 675x, a temperature can
be found at which the bulk chemical composition of the condensate comes very close to the average composition of L-Group chondrite falls from
Jarosewich (1990). In the following comparison, sufficient metal of the same composition as that in Jarosewich’s average L-Group chondrite has been
added to his average L-Group chondrite bulk composition to yield the same atomic Fe/Si ratio as in H-Group chondrites. At 1330K, the condensate
contains 16.8 (16.1) wt % metal, 12.9 (13.2) % FeO, 5.5 (5.3) % FeS, 34.0 (36.3) % SiO2 and 24.5 (22.6) % MgO. In both cases, the
MgO/SiO2 ratio of the condensate is higher than in the chondrite due to the fact that the relative abundances of non-volatile elements in
the model system are those of C1 chondrites, which are known to have a higher atomic Mg/Si ratio than ordinary chondrites. Nevertheless, the close
correspondence in composition between the predicted condensates and the chondrite averages serves to emphasize the point that the distribution of
iron between metal, silicate and sulfide in ordinary chondrites could have been established during high-temperature condensation in a dust-enriched system.
In Fig. 9a and b, the bulk chemical composition of the total condensate is plotted as a function of temperature at a
Ptot of 10-3 bar and a dust enrichment of 560x. Features of this diagram which are
common to condensation at all dust enrichments are the early entry of Al, Ca and Ti relative to Mg and Si, as well as the relatively late entry of
Na, K and Mn into the condensates. Features specific to condensation at this dust enrichment are the relative proportions of metal, FeO and sulfide
as a function of temperature. This particular dust enrichment was chosen because it yields a single temperature at which the distribution of Fe
between metal, sulfide and silicate matches closely the distribution found in H-Group ordinary chondrites and results in a bulk chemical composition
very close to the average of those meteorites. For example, at 1310K, the total condensate and, for comparison (brackets), the average H-Group chondrite
fall from Jarosewich (1990) contains 18.8 (17.8) wt % Fe+Ni+Co metal, 10.2 (10.3) % FeO, 5.9 (5.4) % FeS, 34.2 (36.6) % SiO2
and 24.6 (23.3) % MgO. Similarly, at the same Ptot and a slightly higher dust enrichment of 675x, a temperature can
be found at which the bulk chemical composition of the condensate comes very close to the average composition of L-Group chondrite falls from
Jarosewich (1990). In the following comparison, sufficient metal of the same composition as that in Jarosewich’s average L-Group chondrite has been
added to his average L-Group chondrite bulk composition to yield the same atomic Fe/Si ratio as in H-Group chondrites. At 1330K, the condensate
contains 16.8 (16.1) wt % metal, 12.9 (13.2) % FeO, 5.5 (5.3) % FeS, 34.0 (36.3) % SiO2 and 24.5 (22.6) % MgO. In both cases, the
MgO/SiO2 ratio of the condensate is higher than in the chondrite due to the fact that the relative abundances of non-volatile elements in
the model system are those of C1 chondrites, which are known to have a higher atomic Mg/Si ratio than ordinary chondrites. Nevertheless, the close
correspondence in composition between the predicted condensates and the chondrite averages serves to emphasize the point that the distribution of
iron between metal, silicate and sulfide in ordinary chondrites could have been established during high-temperature condensation in a dust-enriched system.
|