Condensation in Dust-enriched Systems, by D.S. Ebel and L. Grossman,
![]() Geochimica et Cosmochimica Acta, 1999
Geochimica et Cosmochimica Acta, 1999
|
|
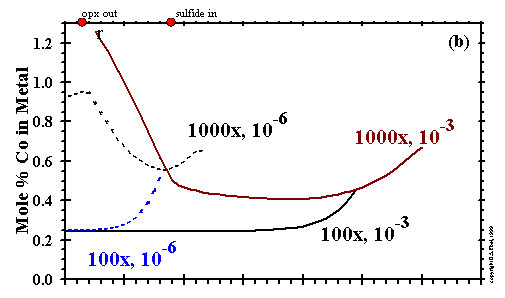 The concentrations of Ni, Co and Cr in the metallic nickel-iron alloy at various combinations
of Ptot and dust enrichment are shown in Figs. 14a, b and c, respectively.
Under all conditions shown, Ni and Co are slightly more refractory and have slightly steeper condensation curves than Fe.
This leads to high concentrations of Ni and Co in the first-condensing alloys, steadily declining concentrations of Ni and Co
with falling temperature as condensation of slightly less refractory Fe dilutes the previously condensed Ni and Co, and
finally a leveling off of the Ni and Co contents when all three elements are totally condensed.
The concentrations of Ni, Co and Cr in the metallic nickel-iron alloy at various combinations
of Ptot and dust enrichment are shown in Figs. 14a, b and c, respectively.
Under all conditions shown, Ni and Co are slightly more refractory and have slightly steeper condensation curves than Fe.
This leads to high concentrations of Ni and Co in the first-condensing alloys, steadily declining concentrations of Ni and Co
with falling temperature as condensation of slightly less refractory Fe dilutes the previously condensed Ni and Co, and
finally a leveling off of the Ni and Co contents when all three elements are totally condensed.
|
 At still lower temperatures in the more oxidizing cases, 1000x at 10-3 and
10-6 bar, Ni and Co contents begin to rise very gradually with falling temperature
due to oxidation of the Fe component of the alloy. At 1380K at 1000x and 10-3 bar,
the Ni and Co contents of the alloy begin to rise very sharply with falling temperature due to reaction of gaseous
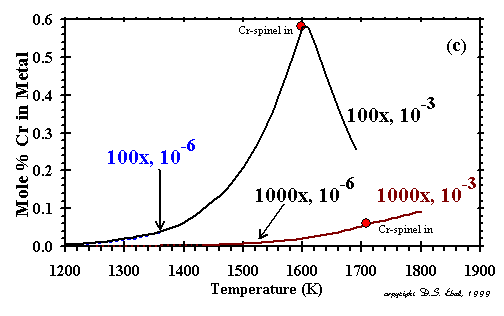
sulfur with the Fe component of the alloy to form pyrrhotite. Under oxidizing conditions, Cr is slightly more refractory
than Fe and, like Ni and Co, falls steadily in concentration with falling temperature. Under more reducing conditions, however,
the behavior of Cr is completely different. At 100x and 10-3 bar, Cr is slightly less
refractory than Fe, its concentration in the metal increases sharply with falling temperature in the high-temperature alloys,
and only reverses itself below the formation temperature of Cr-spinel, which extracts Cr from the metal alloy.
The increase in Cr content with falling temperature is not seen at 100x and 10-6 bar
because most of the Cr has already condensed as Cr-spinel at a higher temperature than that where the metal alloy
begins to condense. While high Si concentrations in metallic nickel-iron alloys can result from condensation from
gases more reducing than a gas of solar composition, XSi is
always < 10-4 in the systems considered in this work.
At still lower temperatures in the more oxidizing cases, 1000x at 10-3 and
10-6 bar, Ni and Co contents begin to rise very gradually with falling temperature
due to oxidation of the Fe component of the alloy. At 1380K at 1000x and 10-3 bar,
the Ni and Co contents of the alloy begin to rise very sharply with falling temperature due to reaction of gaseous
sulfur with the Fe component of the alloy to form pyrrhotite. Under oxidizing conditions, Cr is slightly more refractory
than Fe and, like Ni and Co, falls steadily in concentration with falling temperature. Under more reducing conditions, however,
the behavior of Cr is completely different. At 100x and 10-3 bar, Cr is slightly less
refractory than Fe, its concentration in the metal increases sharply with falling temperature in the high-temperature alloys,
and only reverses itself below the formation temperature of Cr-spinel, which extracts Cr from the metal alloy.
The increase in Cr content with falling temperature is not seen at 100x and 10-6 bar
because most of the Cr has already condensed as Cr-spinel at a higher temperature than that where the metal alloy
begins to condense. While high Si concentrations in metallic nickel-iron alloys can result from condensation from
gases more reducing than a gas of solar composition, XSi is
always < 10-4 in the systems considered in this work.
|