Heteroptera: An Overview of the Classification and Morphology
The following summary is designed to provide context for the Plant Bug Planetary Biodiversity Inventory project, by placing the Heteroptera within the larger monophyletic group Hemipera and the Miridae within the Heteroptera. Much of the information presented is derived from the more detailed summaries provided in CSIRO (1991) and Schuh and Slater (1995) with citations from some additional specialized sources.
To clarify potential confusion that might arise concerning terminology of higher group names, it should be noted some authors continue to use the term Homoptera in reference to all hemipterans other than true bugs. Nonetheless, it is now well demonstrated that Homoptera is simply a grouping of convenience and has no standing in a classification that contains only natural (monophyletic) groups. Furthermore, North American authors, in particular, long used the term Hemiptera in the same sense that European authors used the term Heteroptera. This usage was unfortunate for historical reasons, as well as that it left in doubt the name for the larger group that contained all taxa with the sucking mouthparts distinctive to the Hemiptera, and most importantly because it implied a classification that obscured the true nature of relationships among the groups of organisms concerned. The use of Hemiptera as equivalent to Heteroptera has now been largely–if not totally–abandoned.
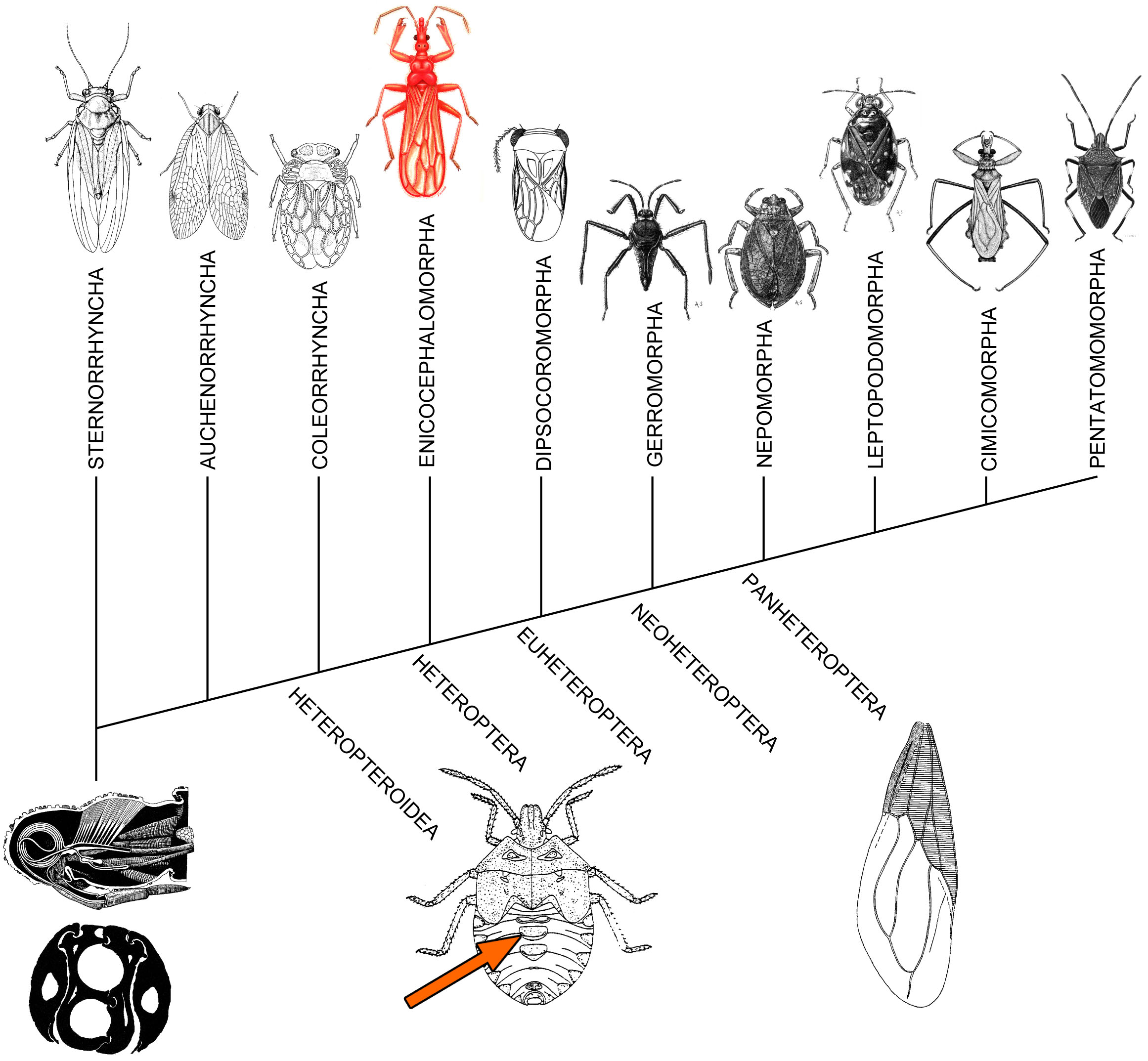
Each grouping shown in the diagram of relationships–whether terminal or inclusive–is discussed in turn. Characters distinctive to the groups, as well as some other features, are shown in boldface in the following paragraphs.
Hemiptera This grouping was first recognized by Linnaeus in the Systema Naturae of 1758. It is defined as monophyletic by its possession of distinctive mouthparts in which the labium assumes the form of a sheath surrounding the elongate, slender mandibles and maxillae. The maxillae are modified into a pair of concentric tubes forming the salivary and food canals; the mandibles lie external to the maxillae, are coupled to them, and serve a cutting function when introducing the mouthparts into the food source. The maxillary and labial palpi are completely lost in the Hemiptera. Functioning of this feeding method requires that food be liquid or be suspended in a liquid medium. Saliva, which may form a variety of functions including hystolysis, paralysis, or anticoagulant–among others–passes through the salivary canal to the food source. The liquid food is then drawn into the insect gut through the sucking action of a cibarial pump.
Sternorrhyncha The basal grouping within the Hemiptera includes scale insects (Coccina), aphids (Aphidina), white flies (Aleyrodina), and psyllids (Psyllina). They are characterized by several features distinctive to them, including absence of vannus and vannal fold in the hind wing (CSIRO, 1991), base of labium in posterior position (opisthognathous) (CSIRO, 1991; Ax, 1999), 2 tarsal segments (Ax, 1999), radius, media, and cubitus fused basally (Ax, 1999).
Auchenorrhyncha + Heteropterodea The following attributes are diagnositic for this group: posterior pronotal lobe covering at least the anterior portion of mesonotum (Ax, 1999); lateral process of [meso]scutellum connected to mesosubalare (Larsen, 1945; Ax, 1999).
Auchenorrhyncha There is disagreement among modern authors on the composition of this group. We use Auchenorrhyncha in the broad sense to include the plant hoppers, or Fulgoroidea, as well as the cicadas, leafhoppers, and frog hoppers, and all of their relatives. Auchenorrhyncha are defined by the possession of a tymbal acoustic system (CSIRO, 1991) and an aristoid antennal flagellum (CSIRO, 1991).
Heteropterodea This grouping, sometimes referred to as Heteropteroidea, comprises hemipteran taxa that are capable of folding their wings over the dorsal surface of the body in an overlapping fashion. The group was first recognized by Schlee (1969) after a period of confusion in which the Coleorrhyncha has been placed in the "Homoptera" and the Heteroptera. Characters arguing for the monophyly of the Heteropterodea include: head prognathous (Ax, 1999), tergites flattened, rather than convex, resulting in the characteristic flat body cross section, in contrast to the circular crossection in Sternorrhyncha and Auchenorrhyncha (Schlee, 1969), and horse-shoe shaped sclerite at base of aedeagus (Schlee, 1969).
Coleorrhyncha Members of this obscure, but phylogenetically pivotal group, are placed in the single family Peloridiidae. They have become known as moss bugs for their habit of feeding on mosses. Their modern distribution in the cool temperate forests of New Zealand, Australia, and far southern South America suggests a remnant of a once more widespread group, and also makes their discovery by the ordinary observer quite unlikely. The Peloridiidae are characterized by the novel structure of the frontal lobes of the head (Schlee, 1969), 2-segmented tarsi (Ax, 1999), cryptic insertion of the antenna (Ax, 1999). Their possession of many characters also found in the Auchenorrhyncha led earlier authors to place the peloridiids in the "Homoptera".
Heteroptera
Heteroptera are unequivocally diagnosed by their possession of 1-4 pairs of scent glands located in, and opening between, abdominal terga 3-7 in the nymphs. The labium is inserted anteriorly on the head and a distinct gula is always present. Contrary to earlier diagnoses, the presence of scent glands in the adult metathorax does not form a diagnosis for Heteroptera including Enicocephalomorpha. Nonetheless, the acone ommatidium with an open rhabdom apparently does (Fischer et al. 2000), as may the presence of a frenum that attaches the posterior margin of the front wing to the [meso]scutellum.
The Heteroptera, or true bugs, are generally treated as a suborder, as are the Sternorrhyncha, Auchenorrhyncha, and Coleorrhyncha. The terminal groupings with the Heteroptera are referred to as infraorders by most modern authors, a terminology which we shall follow. The majority of these subdivisions, with a composition similar to that used here, were codified in a small, unillustrated paper published by Leston, Pendergrast, and Southwood (1954). These authors introduced the terms Cimicomorpha and Pentatomomorpha in the first formal attempt to recognize natural groups within the Geocorisae of older authors, grouping that include all terrestrial true bugs, but which had no unique defining features.
The influence of the Leston, Pendergrast, and Southwood paper was profound, other authors adopting these groups and applying the "morpha" nomenclature to additional higher groups in the Heteroptera, although not without some variant spellings along the way. More importantly, their work spurred the attempt to document the monophyly of higher groups within the Heteroptera, with the eventual recognition of 7 such terminal groups, as discussed by Stys and Kerzhner (1975).
The first documented synapomorphy scheme for the 7 heteropteran infraorders was that of Schuh (1979). The character data were drawn mostly from information assembled by Cobben (1978). Stys (1985a) provided a set of names for the basal inclusive groups on the cladogram. Little new evidence for higher-group relationships within the Heteroptera was adduced after the work of Schuh (1979), until the publication of 18s nuclear rDNA sequences by Wheeler et al. (1993). Their scheme is accompanying figure.
Enicocephalomorpha
Features of this seldom seen group of true bugs have not figured prominently–if at all–in many classificatory schemes of the Heteroptera, or their morphology was incompletely appreciated, leading to their placement with the Reduviidae, the latter being members of the infraorder Cimicomorpha. Nonetheless, the Enicocephalomorpha have come to occupy the pivotal basal position in current hypotheses of heteropteran phylogenetic relationships. The group is biologically interesting for its habit of forming single-sex mating swarms. It is through the observation of these swarms, or the attraction of individuals to lights, that the majority of known specimens have been collected. Most of the modern work on morphology and higher classification of the unique-headed bugs has been done by Pavel Stys.
Enicophalomorpha are diagnosed by the division of the head into anterior and posterior lobes by way of a postocular constriction. The foretibia is dilated, armed with 1 or 2 clusters of spiniform setae, and can be opposed with 1 or 2-segmented tarsus. The male genitalia are always symmetrical and possess paired genital plates similar to the condition seen in the Auchenorrhyncha. The female subgenital plate is formed of abdominal sternum 8 rather than 7, as is the case in all other Heteroptera.
Euheteroptera
Diagnosed by the subgenital plate in the female being formed by abdominal sternum 7 rather than 8.
Dipsocoromorpha
Members of this group, although lacking a generally accepted common name, might be referred to as minute tropical litter bugs. Like the Enicocephalomorpha, their cryptic habits and very small size cause them to be seldom seen by most entomologists.
Several features are distinctive to the group and suggest monophyly. The antennae are flagelliform, as in the Enicocephalomorpha, but have the distinctive feature of having segments 1 and 2 reduced in length (except the Stemmocrytpidae), a condition seen elsewhere in the Heteroptera in very few taxa. The male genitalia are assymmetrical, with the exception of some members of the Ceratocombidae, and have the unusual property of functionally including in many taxa what are–in most other bug lineages–pregenital segments (but see below under Nepomorpha). Most, if not all, members of the group possess "adhesive pads" on the hind coxae. In what apparently represents a primitive condition in the Heteroptera, the pretarsus of at least one pair of legs bears large dorsal and ventral arolia. Unlike other Heteroptera, the condition of the pretarsus may vary from leg to leg, and between the sexes. Other potential characters may include the articulated, paramere-like laterotergites on pregenital segments in males (CSIRO, 1991) and the characteristic spermathecal pump (CSIRO, 1991).
Neoheteroptera
The presence of more than 5 compound eye facets in first instar nymphs of most taxa was treated as a synapomorphy for this group by Schuh (1979) and Wheeler et al. (1993) on the basis of data originally developed by Cobben (1978).
Gerromorpha
The semiaquatic bugs, as members of this group are commonly known, have the distinctive ability to walk–even complete their entire life cycle–on the water surface. They have been intensively studied by the late Nils Moller Andersen, who produced a body of seminal works clarifying morphology and outlining phylogenetic relationships within the group.
Features diagnostic for the Gerromorpha include–among others–the presence of 3 or 4 pairs of trichobothria on the head inserted in deep pits and distinctive structures known as peg plates (or sieve pores) that are widely distributed on the body surface. The relatively basal position of the Gerromorpha within the Heteroptera is maintain by the structure of the wings, which is not developed into a "hemelytron", whereby the anterior portion of the wing is coriaceous and the posterior portion is completely membranous.
Panheteroptera
The well developed hemelytron is the most obvious feature diagnosing this group.
Nepomorpha
The true water bugs, like the Gerromorpha, have long been recognized as a group. What has changed is that many modern authors do not consider them to occupy a basal position in the phylogenetic scheme for true bugs as was assumed by many earlier students of the group. This group contains some of the largest and most conspicuous of true bugs, including giant water bugs and water scorpions. Members of many families are also readily collected because their habitats are easily identified.
The reduced, and usually hidden, antennae are the most obvious feature uniting members of this group. Nonetheless, other features such as scolopophorous organs in the meso- and metathorax also appear to be diagnostic. Many features in the group are associated with the aquatic life style of the majority of the families, but the reduced antennae exist in terrestrial as well as aquatic taxa. In all members except the Nepoidea the genital segments, and sometimes pregenital abdominal segments, are asymmetrical.
Leptopodomorpha + "Geocorisae"
This grouping lacks a formal name. The spermathecal flanges are a potential synapomorphy of the group, although they are only present in Leptopodomorpha and Pentatomomorpha, with their absence in the Cimicomorpha apparently being an evolutionary loss.
Leptopodomorpha
The shore bugs and their close relatives were at one time classed as semiaquatic bugs. Modern phylogenetic studies have revealed, however, that they are actually more closely related to the "Geocorisae" than to the Gerromorpha. Features distinctive to the group include the grasping apparatus between abdominal segments 2 and 3 in males which is used to hold the forewing of the female during copulation and the reduction of the parempodia in most taxa.
"Geocorisae"
This name is now used for a grouping of significantly altered composition from its original application. There are no unequivocal morphogical features that serve as diagnostic characters of this group. Nonetheless, the ventral arolium is apparently reduced in all life stages of this group, contrary to the situation found in the Leptopodomorpha.
Cimicomorpha
This grouping contains the two largest families of true bugs, the assassin bugs and the plant bugs. Group-defining characters include the reduction of the median spermatheca to a non-functional state or to a vermiform gland and the aeropyles and micropyles in the eggs arranged in a ring outside the operculum.
Pentatomomorpha
The flat bugs, seed bugs, leaf-footed bugs, and stink bugs are some of the most distinctive groups within the Pentatomomorpha. All members except the Aradidae + Termitaphididae possess 2 or more trichobothria on several abdominal segments, a feature that caused Tullgren in 1918 to refer to these taxa collectively as the Trichophora. Leston, Pendergrast, and Southwood realized that many features occuring in the Trichophora also occur in the Aradidae and united these taxa within the Pentatomomorpha.
Features distinctive to the Pentatomomorpha as a whole include the characteristic large pulvilli attached to the base of the claw that are present in all but a very few of the nearly 15,000 species, the gastric caeca associated with the midgut of most taxa, and the cephalic pole of the eggs with micropylar processes.
References
Ax, P. 1999. Das System der Metazoa II. Ein Lehrbuch der Phylogenetischen Systematik. 1999. Koeltz Scientific Books, Koenigstein. 384 pp.
Cobben, R. H. 1978. Evolutionary Trends in Heteroptera. Part II. Mouthpart-structures and feeding strategies. Agricultural University, Wageningen.
CSIRO. 1991. Insects of Australia. Volume 1. Melbourne Univeristy Press, Melbourne.
Fischer, C., Mahner, M. & Wachman, E. 2000. The rhabdom structure in the ommatidia of the Heteroptera (Insecta), and its phylogenetic significance. Zoomorphology 120: 1-13.
Larsen, O. 1945. Der Thorax der Heteropteren. Kelett und Muskulatur. Acta Univ. Lund. N.F., Avd. 2 41(3):1-96.
Sclee, D. 1969. Morphologie und Symbiose; ihre Beweiskraft fur die Verwandtschafftsbeziehungen der Coleorrhyncha (Insecta, Hemiptera). Stuttgarter Beitraege Naturkunde 210: 1-27.
Schuh, R. T. 1979. [Review of] Evolutionary Trends in Heteroptera. Part II. Mouthpart-structures and feeding strategies. Systematic Zoology 28: 653-656.
Schuh, R. T. and J. A. Slater. 1995. True Bugs of the World (Hemiptera: Heteroptera). Classification and Natural History. Cornell University Press, Ithaca, New York. xii + 338 pp.
Stys, P. and I. M. Kerzhner. 1975. The rank and nomenclature of higher taxa in the Heteroptera. Acta Entomologica Bohemoslavica 72: 65-79.
Wheeler, W. C., R. T. Schuh, and R. Bang. 1993. Cladistic relationships among the higher groups of Heteroptera: congruence between morphological and molecular data sets. Entomologica Scandanavica 24; 121-137.