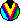
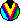
The VAPORS condensation code is the result of work by Denton Ebel, and continues work begun in 1995 in the laboratory of Dr. Lawrence Grossman at the University of Chicago, Department of Geophysical Sciences. The code computes the equilibrium thermodynamic state in a system consisting of 23 elements at the high temperatures (1000 - 2400 K) and low pressures (PTOT < 1.0) appropriate to the solar nebula. A large variety of gas molecules, and solid and liquid condensate phases are considered in the calculation of equilibrium in these systems.
The VAPORS code differs from previous condensation codes in by including internally consistent models for silicate mineral solid solutions and silicate liquid. The MELTS silicate liquid model is fully implemented in the VAPORS code.
We recently applied this model to the calculation of condensation in the plume expected to result from the impact of a 10 kilometer diameter meteorite in the Yucutan peninsula at Chicxulub, 65 million years ago. Reentry of matarial from this impact was the direct cause of a mass extinction (see this page: KT-1.html for more details).
Condensation occurs when a low density cloud of dust
and gas is heated to a temperature where everything becomes vapor, and is
then allowed to cool. The formation of our solar system occurred by partial
evaporation and recondensation of previously existing dust. Dust, and perhaps
pre-solar grains of larger size, agglomerated into larger mineral grains. The
partial melting, and/or evaporation of these grains, as well as further
condensation from the surrounding gas, led to the formation of small round
objects of 0.1 to 5mm in diameter. These objects make up the bulk of many
meteorites, which aggregated to form planetesimals, and then planets.
Remnants of these processes remain as parts of the meteorites called chondrites.
 Image from Doug Johnstone's web page The image illustrates the center of the Trapezium cluster showing the four massive energetic stars and a number of evaporating proto-planetary disks. This false color mosaic, made by combining multiple Hubble Space Telescope images, was presented to the American Astronomical Society meeting in Toronto, Canada on January 14th, 1997. PHOTO CREDIT: John Bally, Dave Devine, and Ralph Sutherland. This image is shamelessly copied from NASA-STSCI's Orion page, which has great images of the 'stellar nursery' in Orion. |
This image illustrates a dust-rich cloud of gas molecules in Orion, from which numerous protoplanetary disks have been observed to have formed by local collapse. The chemical fate of the material in these disks is the subject of the VAPORS project. |
| --------------- | --------------- |
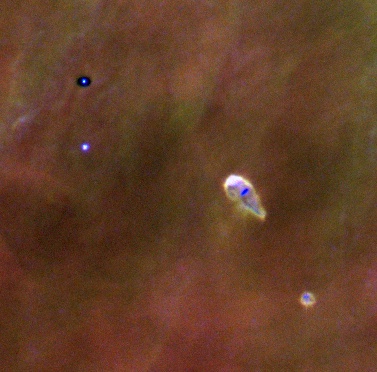 Image from Doug Johnstone's web page This image is shamelessly copied from NASA-STSCI's Orion site. |
Here is a false color image of the teardrop shaped HST 10 star-disk system and immediate neighbors, a silhouetted disk (top left) and a second star-disk system (bottom right). At the center of HST 10 lies a dark nearly edge-on disk with a diameter approximately the same as Pluto's orbit. Surrounding the system is diffuse hot gas which has been evaporated from the disk surface. We are witnessing the destruction of a circumstellar disk which if otherwise left alone would be a strong candidate for producing planets. When a dust-rich region of space collapses due to its own gravity, a proto-sun eventually forms, with a disk of material surrounding it. In this disk, temperatures and pressures increase towards the proto-sun. Temperatures up to 3 A.U. (3 Sun-Earth distances) from the center are predicted to be above the boiling temperatures of most or all of the minerals which would be present as dust. |
| --------------- | --------------- |
|
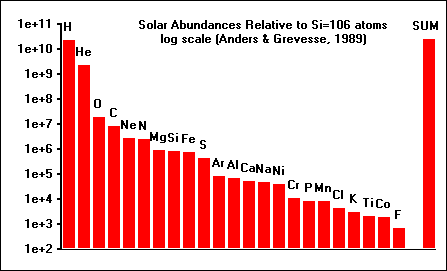
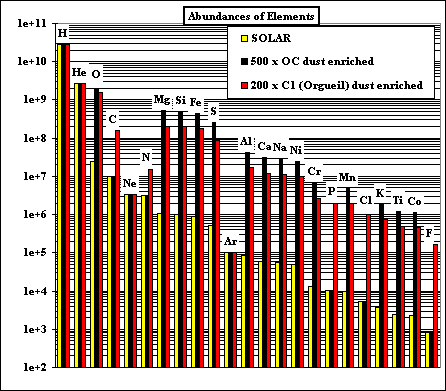
This graph shows the relative abundances, on a log scale,
of 23 of the most abundant elements in our solar system. By far the most
abundant element is hydrogen (H), followed by helium (He). These elements do
not condense in appreciable quantities, but remain gaseous to low temperatures. We are interested in the fate of the elements which make planets, during the pre-planetary stages of the solar system. One way to understand the fate of elements like silicon (Si), aluminum (Al), and magnesium (Mg) is to calculate the equilibrium thermodynamic state of the solar system at the temperatures (1200 - 2500 K) and pressures (PTOT < 1.0) appropriate to the early solar nebula. |
| --------------- | --------------- |
|
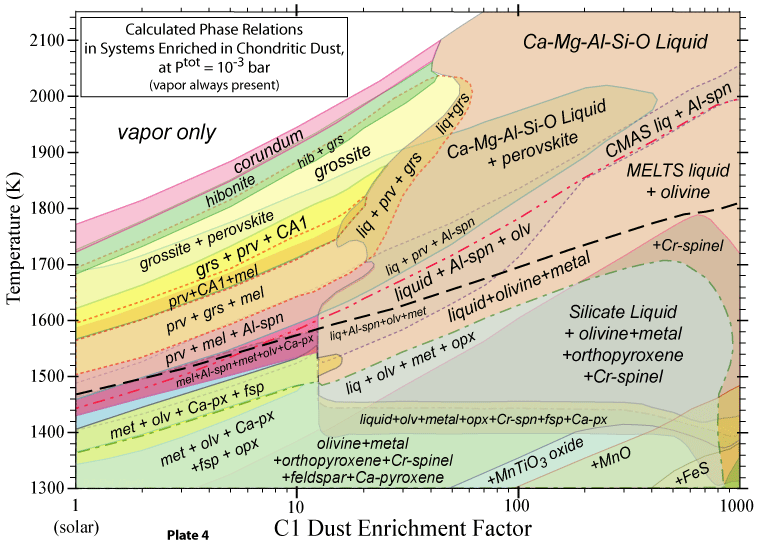
The bulk compositions we investigate are 'solar', corresponding to the accepted
solar abundances of all the elements, and dust-enriched systems. The latter are thought to be likely in
the inner nebular region (< 1 AU), where dust would accumulate relative to gas. Two kinds of dust are considered: OC and C1. The OC dust has the composition of ordinary chondrite, in which all the elements except H, C, N, O, Cl, F and noble gases are totally condensed, and O is condensed as if all the cations are condensed as oxides or halides, except the Fe accounted for by combination with S as FeS. The C1 dust has the composition of the Orgueil carbonaceous chondrite, neglecting the noble gases. Hence the C1 dust is more oxidizing than the OC dust, for the same enrichment factor relative to solar. |
| --------------- | --------------- |
|
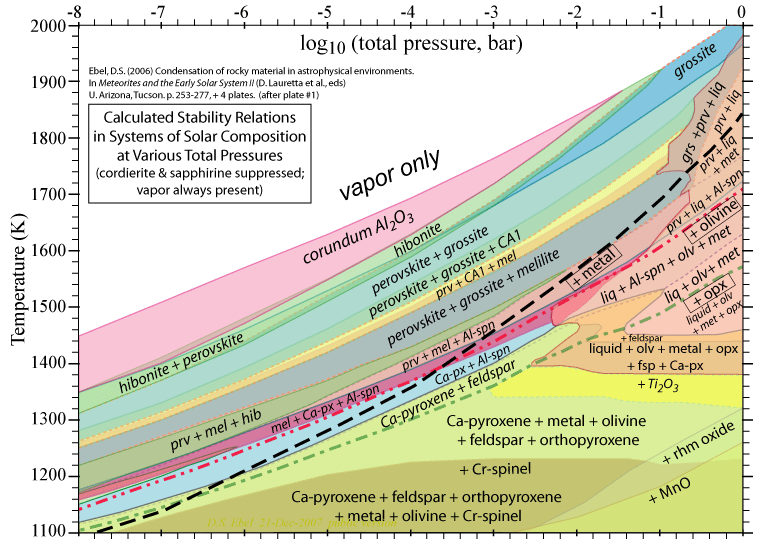
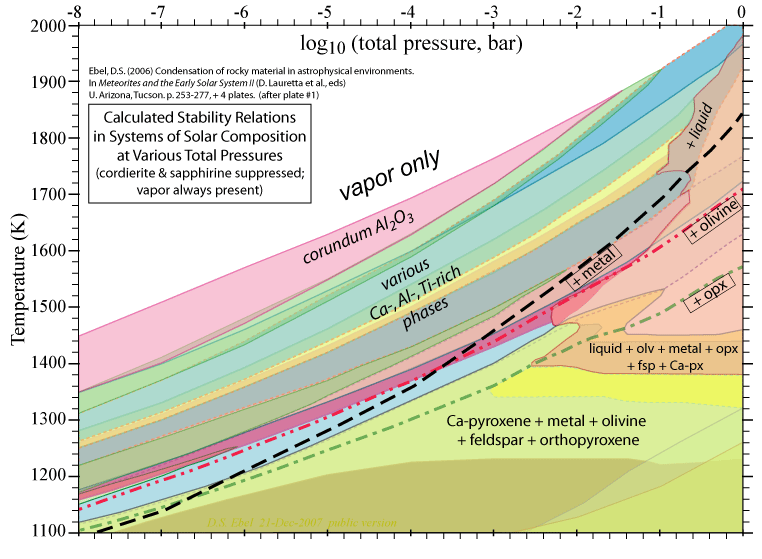
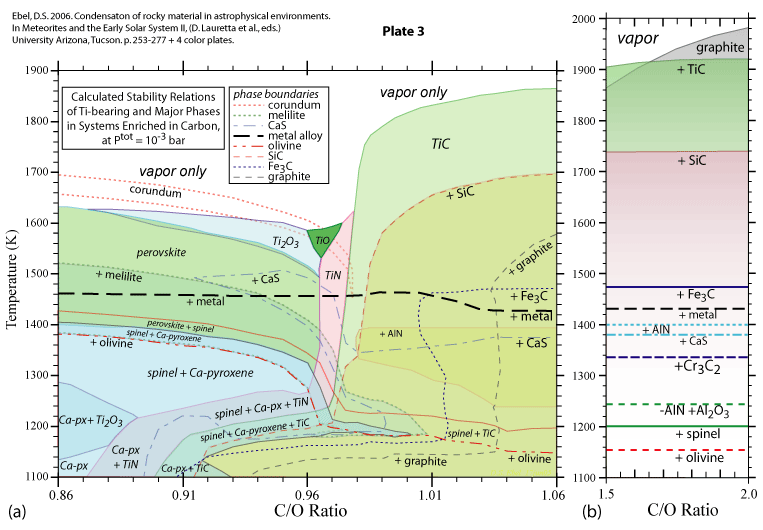
Some of the results of our calculations are illustrated here, however the
dimensions may be slightly distorted due to image reduction techniques. We reported early
results of these calculations at the 28th - 29th Lunar and Planetary
Science Conferences held in Houston in 1997
and 1998,
and at the Meteoritical Society meetings of 1996, 1997
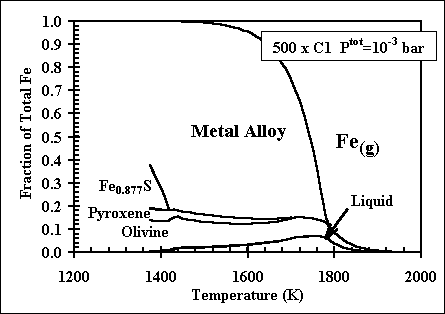
and 1998. More recent, comprehensive results are presented in these papers: Ebel, D.S. 2006. Condensaton of rocky material in astrophysical environments. In Meteorites and the Early Solar System II, (D. Lauretta et al., eds.) University Arizona, Tucson. p. 253-277. Ebel, D.S. and L. Grossman. 2000. Condensation in dust-enriched systems, Geochimica et Cosmochimica Acta, 64: 339-366. Here the distribution of iron between condensed phases is shown, as a dust-enriched gas condenses. The wide stability field of the assemblage silicate Liquid + olivine + metal + (ortho)pyroxene is apparent. cf: Ebel and Grossman (1997) Lunar and Planetary Science XXVIII: 317-318. |
| --------------- | --------------- |
|
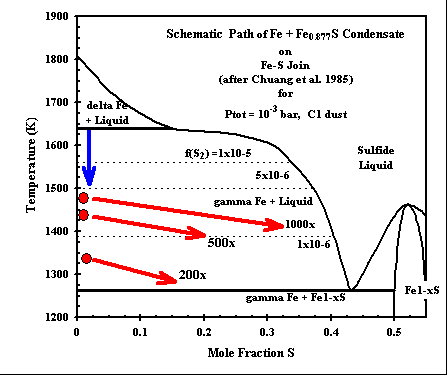
Sulfides of Fe and Ni are found as rims on rounded Fe-Ni alloy grains in many
chondrites. Metal alloy condenses at high temperatures, incorporating nearly 10 wt% Ni. In our calculations
at 500x and 1000x C1 dust enrichment, the solid phase Fe0.877S (pyrrhotite, data from the JANAF tables)
condenses in equilibrium with the metal, and silicate liquid, olivine and orthopyroxene at a temperature
well above the FeS-Fe eutectic, which has heretofore been considered the maximum possible sulfide condensation
tempererature. We do not have a sulfide liquid (Fe-Ni-S) in our calculations yet. But the stability of the solid metal+sulfide assemblage indicates that such a liquid might become stable at a temperature even higher than that indicated here! The bulk (total) Fe-Ni alloy + pyrrhotite composition can be normalized to Fe/(Fe+S), and the trends of such paths are indicated here in the Fe-S binary phase diagram. The path of the total Fe-S condenate indicates that the assemblage METAL ALLOY + LIQUID SULFIDE will be stable over a significant range of temperatures, subject to the unknown effect of sulfur sulubility in coexisting silicate liquid. (cf - Fe-Ni-Cu-S phase diagram) |
| --------------- | --------------- |
|
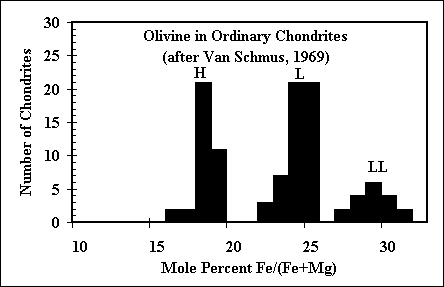
The question of the oxidation state of the environment in which ordinary
chondrite precursors formed remains outstanding in cosmochemistry. Many workers have called on
some kind of dust enrichment, which would cause FeO to condense into olivine well before it does
in a purely solar bulk composition. Our calculations show that if dust enrichment is in fact the
mechanism for an a enhanced oxidation state, then multicomponent silicate liquids are stable in
these systems over a wide temperature range! |