

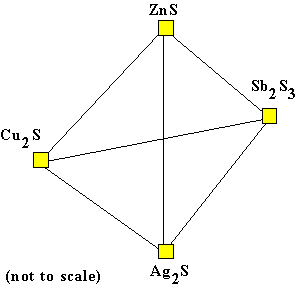
 Quaternary Cu2S - Ag2S - Sb2S3 - ZnS |
The quaternary system Cu2S - Ag2S - Sb2S3 - ZnS
can be illustrated as a tetrahedron. Here the triangle formed by the ternary
Cu2S - Sb2S3 - ZnS is behind everything else, and the
apex Ag2S is toward the viewer. Diagrams of this kind are common in
metallurgy and the geological disciplines of mineralogy and petrology. There are four basic components in the system which may be varied (the apices of the quaternary). In many sulfide ore samples containing zinc (Zn), silver (Ag), copper (Cu) and antimony (Sb) sulfides, the total (bulk) composition of the sample can be described by these four basic components, if others such as FeS are present in negligible amounts. Such a bulk composition plots as a unique point in the tetrahedron. Of course, if one of the four components is not present, the sample will plot on one of the boundaries of the tetrahedron.
|
| ........ | ........ |
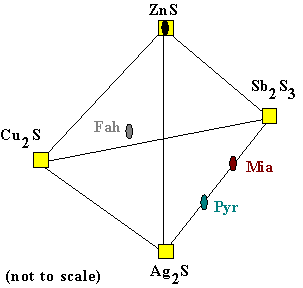 Pure Solids in the Quaternary |
Compositions of specific minerals are now plotted in the
tetrahedron. The oval symbols indicate the (schematic) positions of the ideal,
or pure compositions of the minerals. For example, the mineral 'sphalerite' has composition ZnS, so it is
plotted at the top of the tetrahedron. In this way any mineral which is a linear combination of the four endmembers,
the apices of the tetrahedron, can be plotted. Of course, the pure metal Ag (silver) cannot be plotted, nor can copper (Cu). The mineral 'miargyrite' has the composition AgSbS2. This mineral plots exactly half of the way along the line joining Ag2S and Sb2S3 (the diagram is distorted for clarity). Another mineral plotting on this join is 'pyrargyrite', which has the formula Ag3SbS3. A more complex mineral is 'fahlore' (literally, "gray ore"). It is a complex sulfosalt which is a very important source of silver, worldwide. Here the endmember Cu10Zn2Sb4S13, also known as 'tetrahedrite' is plotted. It plots on the rear triangle of the tetrahedron, and since it contains zinc (Zn), it plots up from the base.
|
| ........ | ........ |
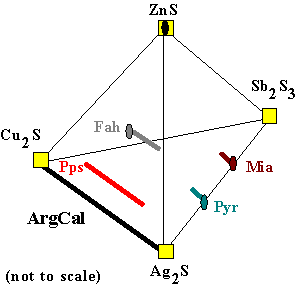 Solid Solutions |
Actually, the phases in question usually contain a mixture
of Ag and Cu in the same sites of their crystal lattices. They are therefore called
solid solutions because they are solutions of two 'pure' compositions. Notice that the solid solution lines are all parallel to the join Cu2S - Ag2S. Two additional phases have been added: ArgCal is the argentite-chalcocite solid solution (Ag,Cu)2S, which is very complex in detail. The other is 'polypearcite', which is a solution of 'pearcite' and 'polybasite', with the general formula (Ag,Cu)16Sb2S11. Of course, in an ore sample, a silver-free fahlore will not coexist with a copper-free pyrargyrite! Instead, Ag and Cu will exchange between the two minerals, and whatever other Ag-Cu sulfosalt is present. In this way an equilibrium will be established, appropriate to the crystal-chemical properties of the solids.
|
| ........ | ........ |
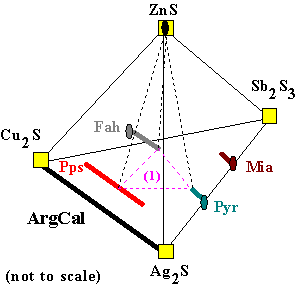 (1) Fah + Pps + Pyr + ZnS |
When four solid phases coexist in this system, their compositions can
be connected as shown here. Any bulk composition which plots in the volume enclosed by the dotted
lines will consist of these four solid solution phases in amounts totalling to the bulk composition. The way that Ag and Cu are distributed between the mineral solutions is shown by the points where the dotted lines intersect the solid solution lines. Although this diagram is not precisely to scale, a larger diagram with gridlines superposed on it would be used to record the compositions found in a particular sample of sulfide ore. This assemblage is what might be found in a system with some ZnS, and not enough antimony (Sb) to stabilize miargyrite instead of polypearcite. A bulk composition plotting near the top of the "1" in (1), would have a small amount of sphalerite (ZnS), and roughly equal amounts of fahlore, polypearcite and pyrargyrite.
|
| ........ | ........ |
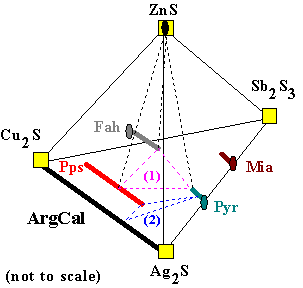 (2) Pps + Pyr + Ag2S |
Of course, maybe an ore sample contains different minerals, such as the assemblage
polypearcite + pyrargyrite + argentite. This assemblage is shown here as (2), in addition
to the assemblage (1). Assemblage (2) might occur in a silver-rich, Zn-free system, with very little Sb or Cu. Such a bulk composition would be very unusual in nature, however the distribution of Ag and Cu between the three phases could easily be explored in the laboratory. Experiments at different temperatures would reveal how the different crystal structures of the minerals accomodate Ag and Cu. This data would be useful in describing the thermodynamic properties of these minerals. Once we have such a description, the compositions observed in nature can be interpreted.
|
| ........ | ........ |
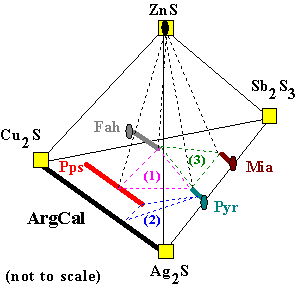 (3) Fah + Pyr + Mia + ZnS |
Here there are three assemblages illustrated. At any particular temperature, pressure,
and bulk composition only one of these assemblages will be the equilibrium one. Once we map how the composition and temperature of ore samples in a mine area change, we can make intelligent decisions about where to look for better ore. Silver ores are usually formed by hot, briny fluids from which the minerals precipitate. Throughout a mine area, the mineral assemblages, bulk ore composition, and the compositions of the mineral solid solutions will reflect the evolution of the ore fluid as it moved away from its source. To minimize the amount of drilling and tunneling necessary, the ability to predict the composition of ore in unexplored areas is required. Understanding the thermodynamic properties of the solids, and having detailed information about ores which have already been drilled, mapped, and analyzed, we can model fluid-rock interaction, and make the required predictions.
|
| ........ | ........ |